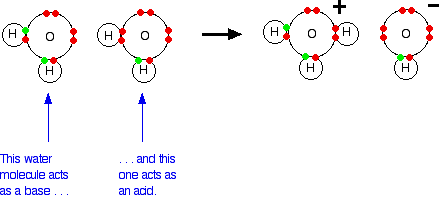
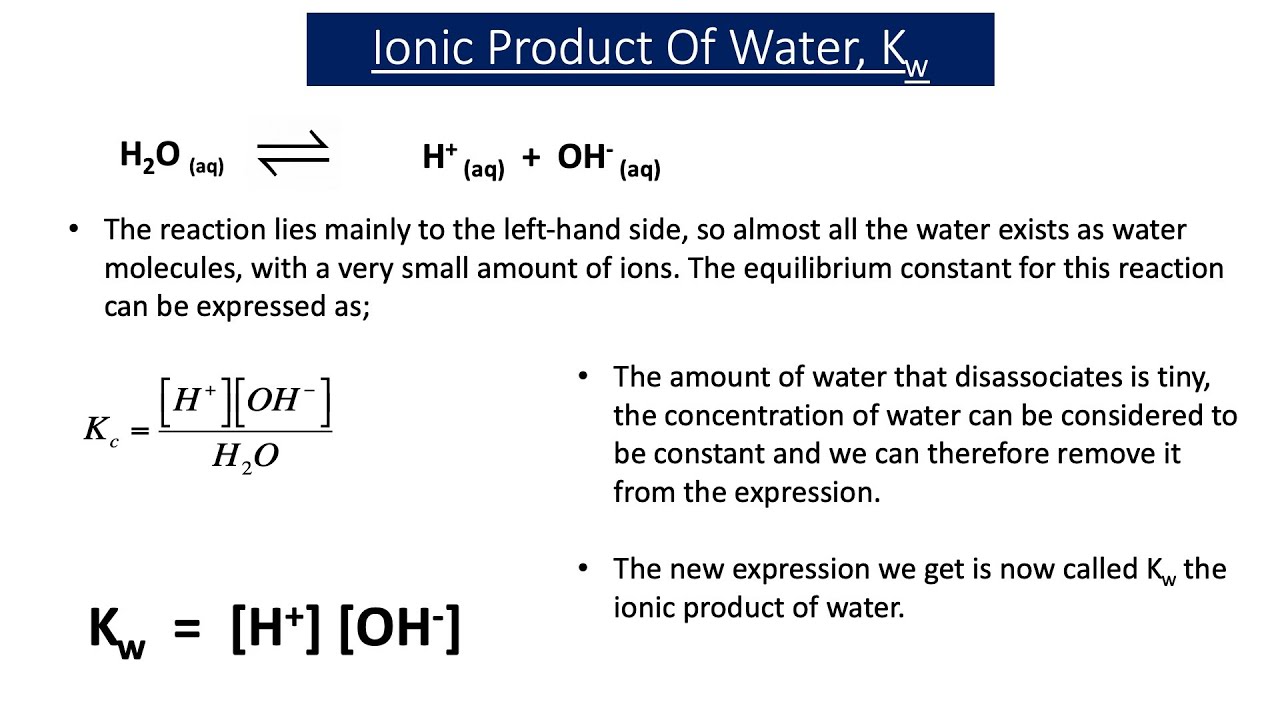
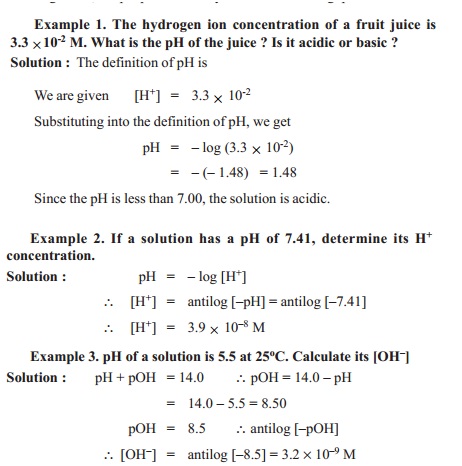
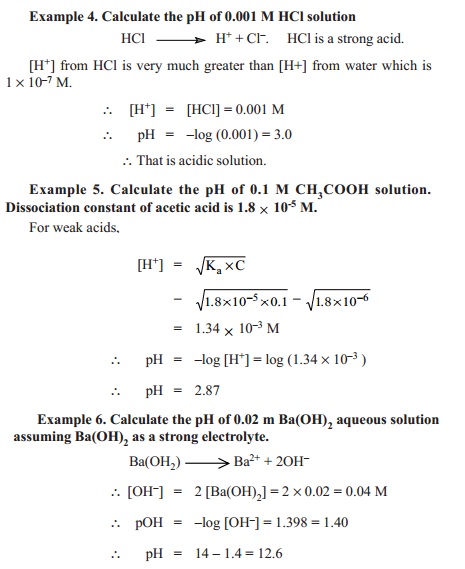
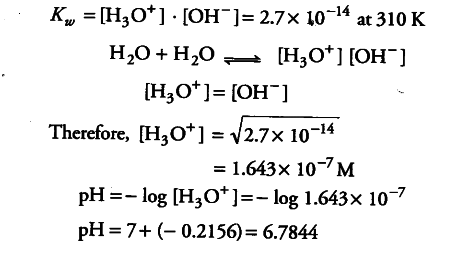![The Ionic Product of Water KwKw. Ionic Product of water, K w Just because a solution contains [H + ] it doesn't necessarily mean it's acidic. All aqueous. - ppt download The Ionic Product of Water KwKw. Ionic Product of water, K w Just because a solution contains [H + ] it doesn't necessarily mean it's acidic. All aqueous. - ppt download](https://images.slideplayer.com/24/7441100/slides/slide_3.jpg)
The Ionic Product of Water KwKw. Ionic Product of water, K w Just because a solution contains [H + ] it doesn't necessarily mean it's acidic. All aqueous. - ppt download

The ionic product of water is 1.0 × 10^-14 at 25^∘C . Assuming the density of water independent from change in temperature, the ionic product of water at 50^∘C will be
![The Ionic Product of Water KwKw. Ionic Product of water, K w Just because a solution contains [H + ] it doesn't necessarily mean it's acidic. All aqueous. - ppt download The Ionic Product of Water KwKw. Ionic Product of water, K w Just because a solution contains [H + ] it doesn't necessarily mean it's acidic. All aqueous. - ppt download](https://images.slideplayer.com/24/7441100/slides/slide_2.jpg)
The Ionic Product of Water KwKw. Ionic Product of water, K w Just because a solution contains [H + ] it doesn't necessarily mean it's acidic. All aqueous. - ppt download

The value of ionic product of water at 393K is | 12 | IONIC EQUILIBRIUM | CHEMISTRY | DINESH P... - YouTube
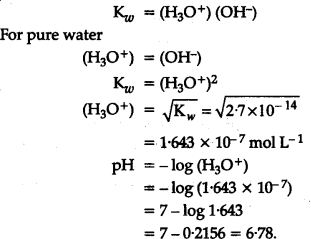
Ionic product of water at 310 K is 2.7 x ${10}^{-14}}$. What is the pH of neutral water at this temp.? - CBSE Class 11 Chemistry - Learn CBSE Forum

The product of molar concentrations of hydrogen ions and hydroxide ions in a 0.01 M aqueous solution of sodium chloride is known as:

please explain each point Q 26 Which of the following is a true statement : (1) The ionisation constant - Chemistry - Chemical Kinetics - 12571323 | Meritnation.com

Ionic product of water at `310 K` is `2.7xx10^(-14)`. What is the `pH` of netural water at this ... - YouTube

The ionic product of water at 100^0C is 55 times than at 25^0 C. The value of pH of water at 100^0C is